
The company also is advancing cerdulatinib, a Syk/JAK inhibitor for the treatment of hematologic cancers.

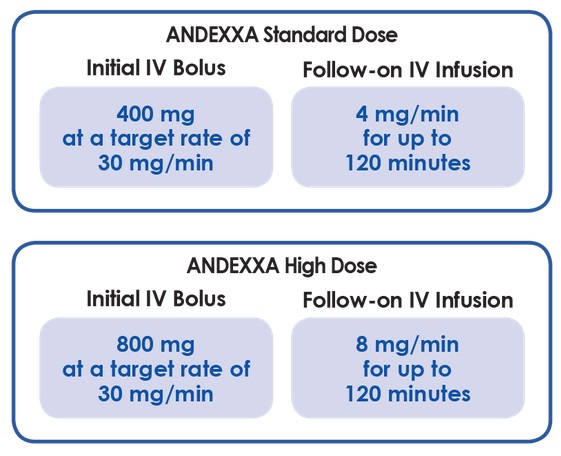
The Company’s two FDA-approved medicines are Andexxa ®, the first and only antidote for patients treated with rivaroxaban and apixaban when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding, and Bevyxxa ® (betrixaban), the first and only oral, once-daily Factor Xa inhibitor for the prevention of VTE in adult patients hospitalized for an acute medical illness. Portola Pharmaceuticals is a commercial-stage biopharmaceutical company focused on the discovery, development and commercialization of novel therapeutics that could significantly advance the fields of thrombosis and other hematologic diseases. Orphan Drug and FDA Breakthrough Therapy designations, and was approved on under the FDA's Accelerated Approval pathway. It is the first and only antidote indicated for patients treated with rivaroxaban and apixaban, when reversal of anticoagulation is needed due to life-threatening or uncontrolled bleeding. If accepted and approved, the PAS will allow for the broad commercial launch of Andexxa in the United States.Īndexxa received both U.S. The PAS has been assigned a Prescription Drug User Fee Act (PDUFA) date of December 31, 2018. Food and Drug Administration (FDA) has acknowledged receipt of the Company’s Prior Approval Supplement (PAS) filing for the large-scale Generation 2 manufacturing process for Andexxa ® .

® (Nasdaq:PTLA) today announced that the U.S. 11, 2018 (GLOBE NEWSWIRE) - Portola Pharmaceuticals, Inc.


 0 kommentar(er)
0 kommentar(er)
